Unit Sales
Expected Gross Profit will be $78.8 million in Year 5.
AFFORDABLE FOR ALL: BALANCING QUALITY WITH ACCESSIBILITY
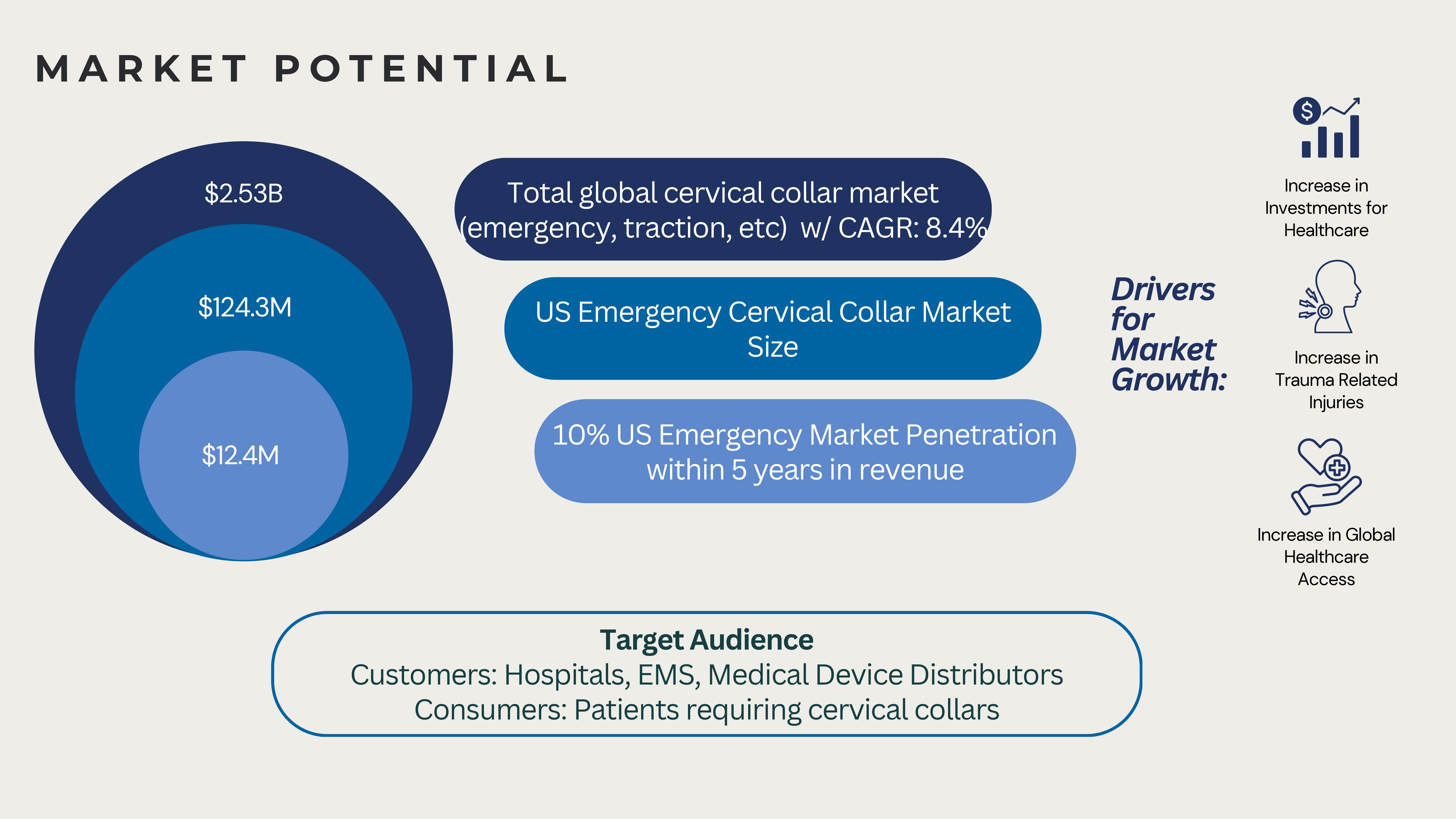
The CerviCollar’s target market includes hospitals, EMS providers, rehabilitation centers, and home healthcare services, settings where rapid and reliable neck stabilization is critical following trauma. Our beachhead market will focus on Level 1–3 trauma centers and EMS agencies in Los Angeles and Orange County, high-volume regions ideal for early adoption, clinical feedback, and visibility. Potential partners who will collaborate to bring our collar to market include medical device manufacturers, distributors, and KOLs such as physicians, nurses, paramedics, and therapists in the healthcare industry through talks, trade shows, and conferences. Licensing the CerviCollar to established medical device companies such as Aspen Medical Products (specializes in long-term settings), Laerdal Medical (common in ambulances), and Össur (all forms of orthotic braces) will facilitate broader distribution and faster market penetration. Surveys and research indicate each ambulance trip requires 2-4 cervical collars[14-16]. Initial commercialization and scaling operations are projected to begin 1.5-2 years after the conclusion of this PoP Grant funding period. This timeline accounts for several critical prerequisites, including startup-scale injection molding, IRB verification, completion of our proposed clinical study, and securing of both manufacturing and supply contracts. Providing CerviCollars to hospitals and ambulances will require prior FDA clearance or approval, which we recognize as an essential regulatory milestone before distribution or sales. The Total Addressable Market (TAM) for cervical collars globally is estimated at $2.97 billion and is expected to grow at an estimated compound annual growth rate (CAGR) of approximately 6.5% to $8.4 billion by 2040. We will start by setting up our Serviceable Obtainable Market (SOM) from key metropolitan areas with the highest rates of motor vehicle collisions and trauma in the US, amounting to $7.2 million in revenue by the end of Year 3. To initiate our beachhead sales, we will provide each hospital with 300 CerviCollars and provide one CerviCollar to 10% of the ambulance trips made in every target region, starting with the Los Angeles area in year 1. From there, we will expand to other major metropolitan regions across the US such as Portland, Kansas City, etc with highest vehicle collision rates, eventually capturing 1% of the national ambulance-based serviceable market. Our international expansion will follow, targeting 1% of the market in Europe and 0.5% in Asia, with market size based on the assumption that each ambulance requires one CerviCollar per trip. The Serviceable Addressable Market (SAM), which encompasses all sales in metro areas globally, will be $1.6 billion by 2040, a feasible goal with global outreach.The CerviCollar will be manufactured through injection molding, achieving the cost of goods sold (COGS) of $5 per unit, enabling a gross profit margin of approximately 85%, taking inflation and material cost fluctuation into account. The COGS not only includes the cost of injection molding, accounting for $0.50 for material, but also packaging and shipping. Importantly, the global cervical collar market is projected to grow steadily over the next 15 years. To bring the product to market, we will utilize a dual-channel strategy: a Business-to-Business (B2B) model, where CerviCollar sells in bulk through top medical distributors to ensure hospitals and EMS providers have consistent, high-quality CerviCollars on hand, and a Direct-to-Consumer (DTC) model, where individuals can purchase CerviCollar directly through our website and retail partners for immediate use in home and community settings.
The images below illustrate Profit Growth spanning from the Serviceable Obtainable Market to the Total Available Market.

Expected Gross Profit will be $78.8 million in Year 5.
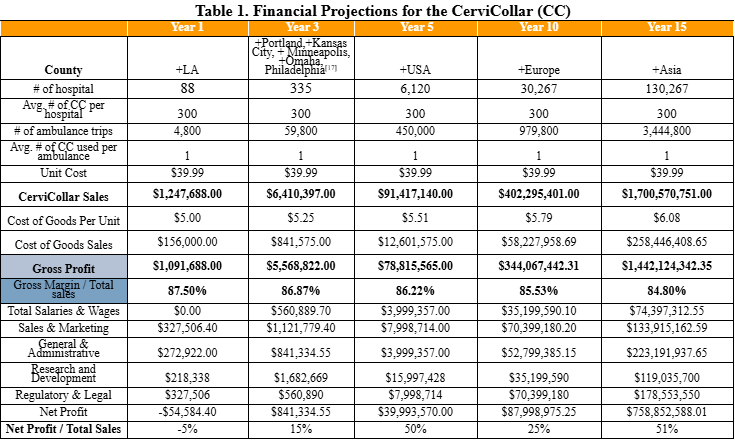
CerviCollar will service the entire United States by Year 5.